featured
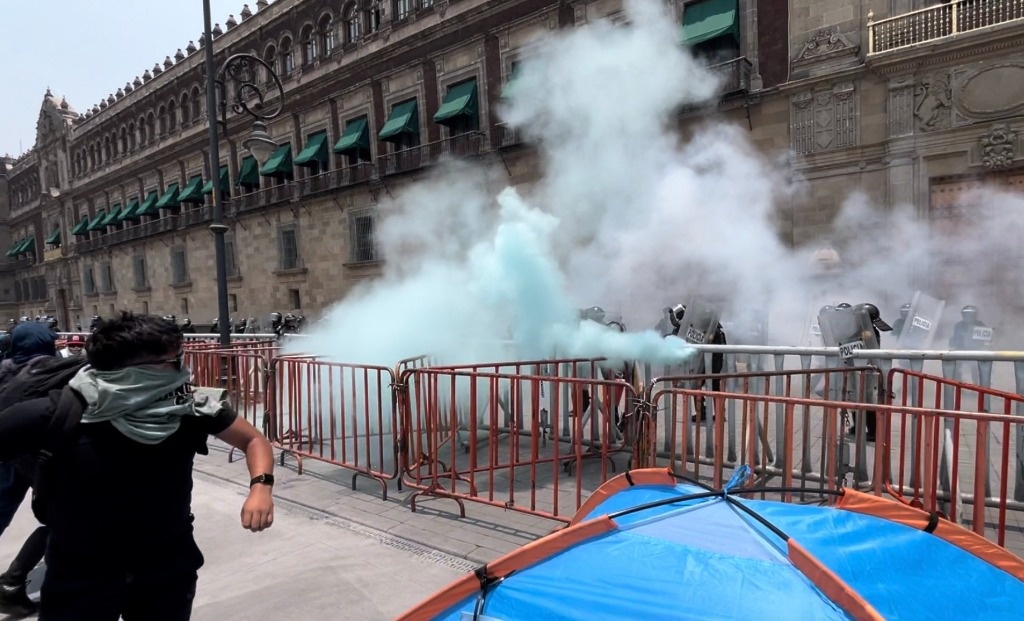
Ayotzinapa college students launch rockets on the Nationwide Palace
With rockets and firecrackers, college students from the Ayotzinapa rural regular college demonstrated yesterday at midday in entrance of the Nationwide Palace in protest of the discharge of eight troopers accused of the pressured disappearance …
Ayotzinapa college students launch rockets on the Nationwide Palace
With rockets and firecrackers, college students from the Ayotzinapa rural regular college demonstrated yesterday at midday in entrance … Read more

Seade: Mexico supplies “monumental safety” to funding from China
Shenzhen. The Mexican ambassador to China, Jesús Seade Kuri, assured that Mexican territory is an funding level with … Read more

Convict Steve Bannon, former Trump advisor, of contempt
Washington. A United States federal appeals court docket confirmed this Friday the four-month jail sentence for contempt of … Read more

To translate, “I have to inhabit the creator’s universe, in his head and pores and skin”
Individuals have the concept that a translator is caught in his home all pale, surrounded by books and … Read more

Mexican pentathletes shut the World Cup with bronze
Mexico Metropolis. The nationwide trendy pentathlon groups, Catherine Oliver and Duilio Carrillo, gained the bronze medal, with a … Read more



