New Study Links Liver Injury Marker to Sepsis Outcomes
Researchers identify key indicator for patient risk
A recent analysis of intensive care unit data has identified a crucial biological marker that may help predict the severity of illness and outcomes for sepsis patients. The research, utilizing a comprehensive dataset, pinpointed a specific calculation that could offer clinicians a clearer picture of patient risk.
Unpacking the Data: Sepsis and Liver Injury Criteria
The study drew upon information from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database, version 3.0. This extensive resource contains detailed clinical data for patients admitted to Beth Israel Deaconess Medical Center from 2008 to 2022. Researchers followed strict protocols, including completing relevant training and obtaining database access approval.
Sepsis was identified using the Sepsis-3 criteria, which involve a suspected or confirmed infection coupled with a significant increase in organ failure scores. Infection and respiratory failure were determined through diagnostic codes, antibiotic use, lab results, and inflammation markers. Notably, a positive culture was not a prerequisite for patient inclusion.
To diagnose sepsis-associated liver injury (SALI), the study applied criteria including an international normalized ratio exceeding 1.5 and total bilirubin levels above 2 mg/dL within the initial 24 hours of ICU admission. Patients with pre-existing liver conditions, such as chronic liver disease or cirrhosis, were excluded to prevent misclassification. This exclusion was based on diagnostic codes related to viral hepatitis, alcohol-related liver disease, autoimmune hepatitis, chronic hepatic failure, and hepatic malignancies.
Further refining the study population, researchers excluded individuals under 18 years old, those with ICU stays shorter than 24 hours, and patients lacking essential data like anion gap or albumin levels within the first day of admission. For patients with multiple admissions, data from only the first instance were used. Ultimately, 443 patients met all inclusion criteria for the analysis.
Extracting Crucial Clinical Information
Data extraction was managed using PostgreSQL and Navicat Premium software, employing SQL to query the MIMIC-IV database. All vital clinical variables, laboratory parameters, and Sequential Organ Failure Assessment (SOFA) scores were gathered within the first 24 hours of ICU admission. The collected information encompassed patient demographics, vital signs, co-existing conditions, laboratory results, treatment details, and survival outcomes, further detailed in Table 1.
Managing Data: Abnormalities and Missing Values
Abnormal variable values were addressed using the `winsor2` command in STATA, applying 1% and 99% cutoffs. Missing data were handled through multiple imputation techniques. Variables with more than 15% missing values were excluded from the analysis.
Calculating Albumin-Corrected Anion Gap (ACAG)
The study calculated the Albumin-Corrected Anion Gap (ACAG) using the formula: ACAG = AG + 2.5 × [4.4 – ALB (g/dL)]. The optimal cutoff value for ACAG, related to 28-day mortality, was determined via receiver operating characteristic curve analysis, using the Youden index to pinpoint the highest diagnostic accuracy.
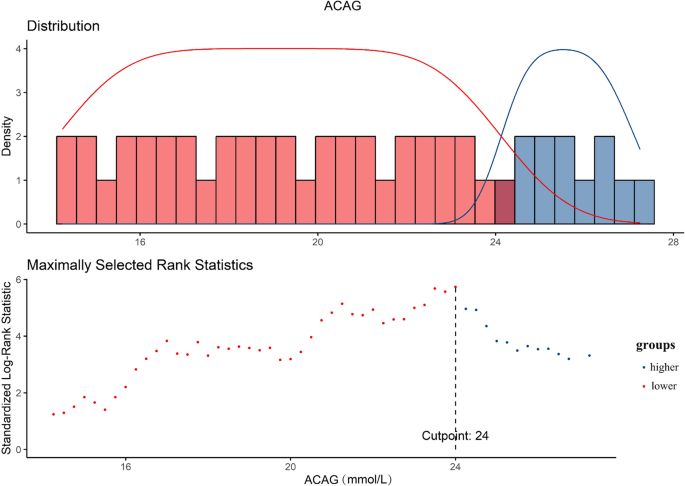
Patients were then categorized into lower and higher ACAG groups based on this established threshold. Researchers tracked mortality outcomes including ICU mortality, in-hospital mortality, and 14-day, 28-day, and 90-day all-cause mortality after ICU admission, using data from the MIMIC-IV database.
Ethical Considerations and Statistical Rigor
The study adhered to the Declaration of Helsinki’s ethical principles. Informed consent was waived by the Beth Israel Deaconess Medical Center’s ethics review board due to the use of de-identified data from the MIMIC-IV database. The board also exempted the study from formal ethical approval, ensuring participant confidentiality.
Statistical analysis involved comparing continuous variables using t-tests or ANOVA for normally distributed data, and the Mann–Whitney U test or Kruskal–Wallis test for non-normally distributed data. Categorical data were analyzed using chi-square tests or Fisher’s exact test.
The association between ACAG and patient prognosis was assessed using Cox proportional hazards models, reporting hazard ratios (HR) with 95% confidence intervals (CI). Three models were developed: an unadjusted model, one adjusted for basic demographics, and a third adjusted for a comprehensive set of clinical factors including creatinine, white blood cell count, platelets, lactate, and various comorbidities and treatments. Kaplan–Meier survival analysis and restricted cubic spline models were employed to examine survival curves and dose-response relationships.
Subgroup analyses were conducted to confirm the prognostic value of ACAG across different patient characteristics, including age, sex, hypertension, diabetes, and respiratory failure. All statistical tests utilized a two-tailed significance level of P < 0.05. Data analysis was performed using R, STATA, and IBM SPSS software.

