New Imaging Technique Offers High-Contrast, real-Time Views of Living Cells
visualizing the intricate details within living cells presents a critically important challenge for biomedical researchers. Traditional optical microscopy often struggles with low contrast and limited resolution, hindering the observation of dynamic cellular processes. Obtaining high-resolution images typically requires techniques that can be damaging to cells or are too slow for real-time monitoring – a critical need for understanding cellular behavior and function.
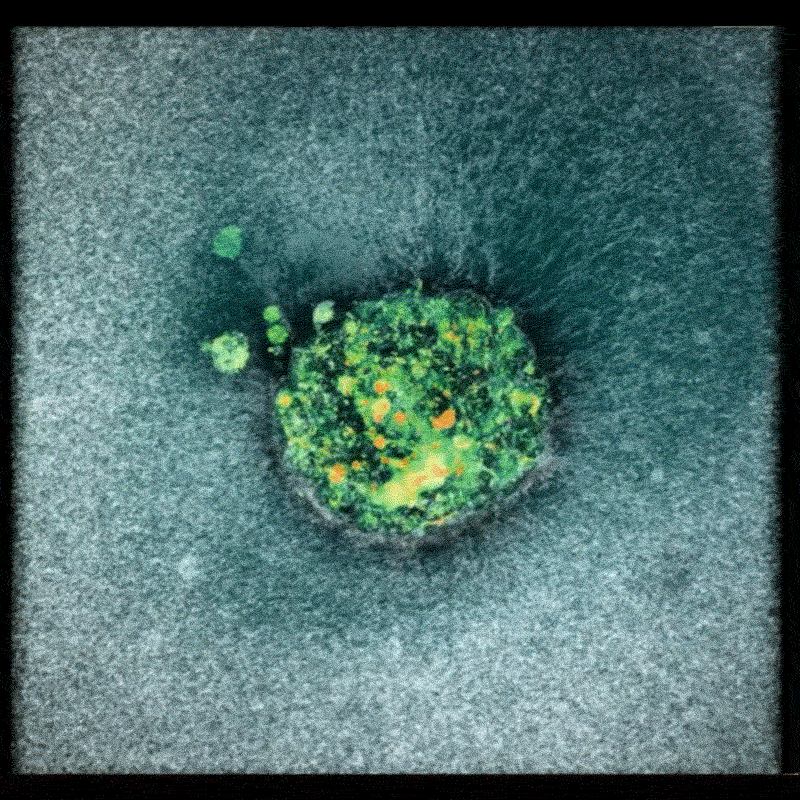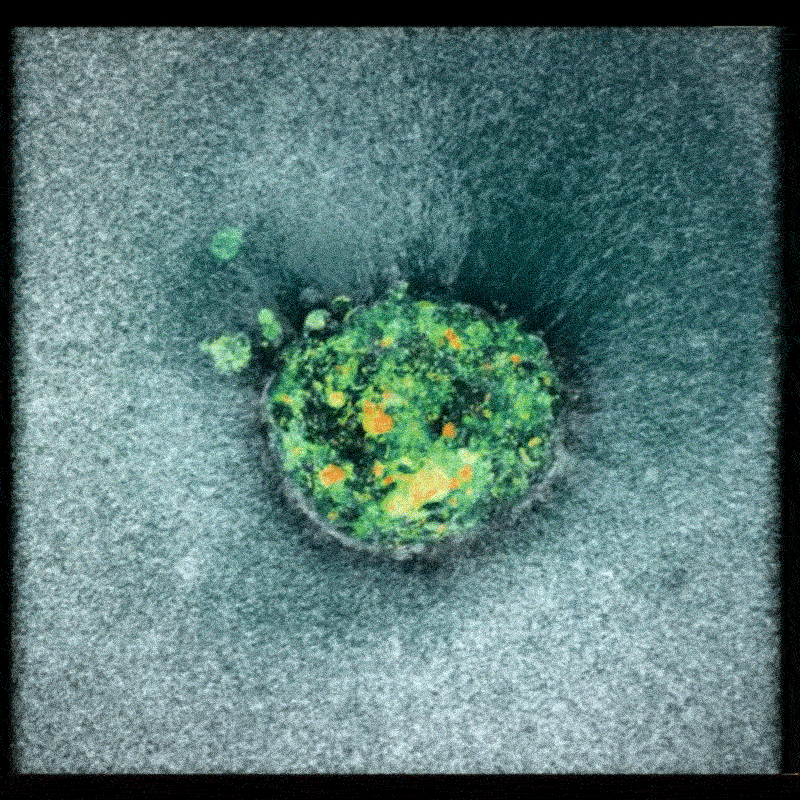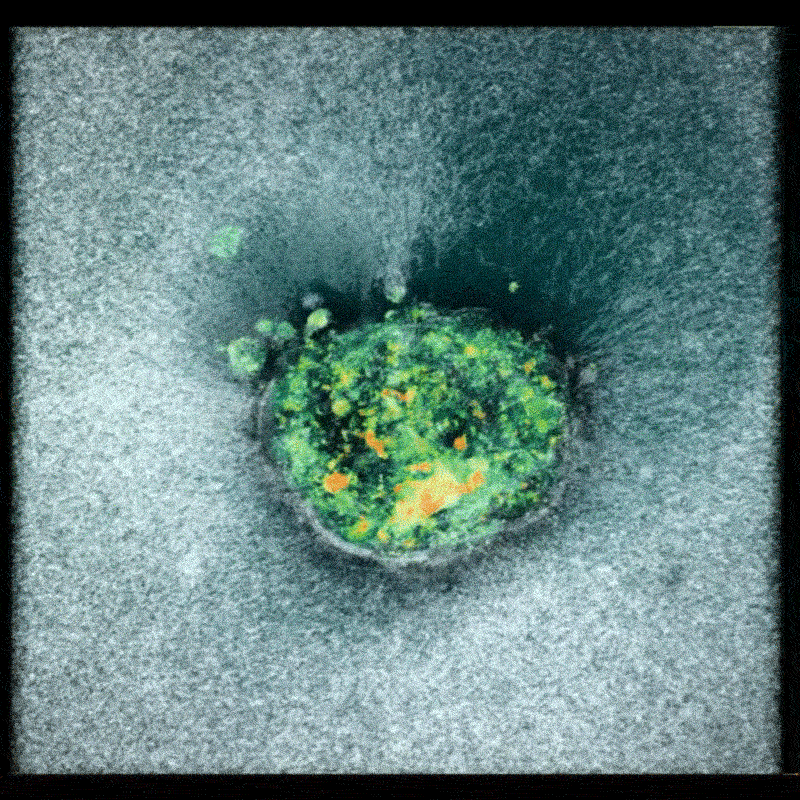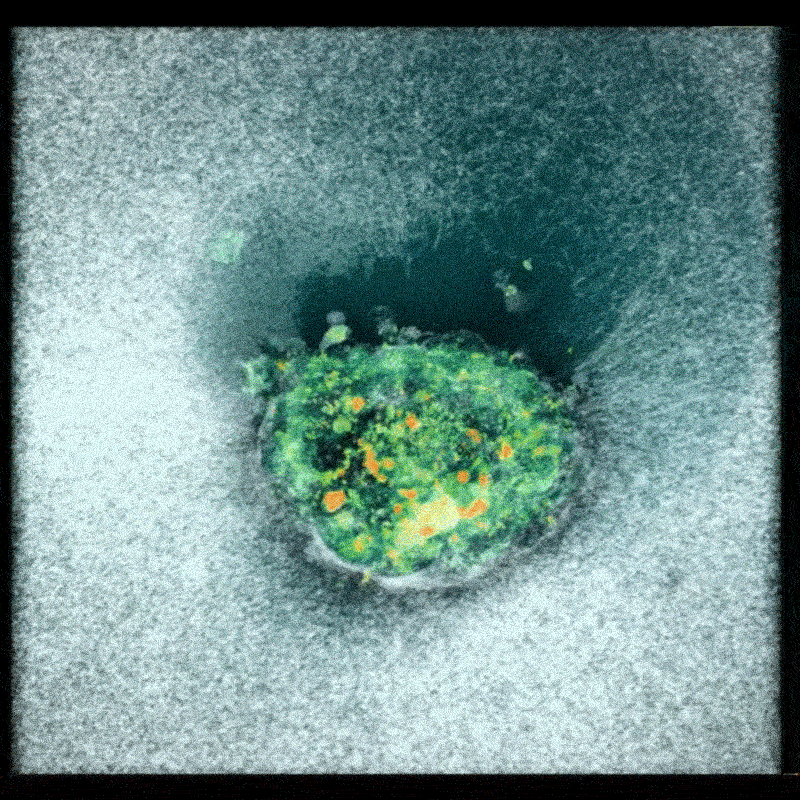
Now, a team of researchers at the Smart Computational imaging Laboratory (SCILab) at Nanjing University of Science and Technology has unveiled a groundbreaking imaging technique poised to overcome these hurdles. Led by Professor Chao Zuo, the team’s work, recently published in iOptics, details a novel approach called dark-field Fourier ptychographic diffraction tomography (DF-FPDT).
DF-FPDT leverages a unique strategy – utilizing “non-matched illumination” – to selectively enhance high-frequency details within the image. This allows for clearer visualization of fine cellular structures without requiring complex hardware modifications or extensive post-processing. Unlike conventional optical diffraction tomography, DF-FPDT effectively filters out low-frequency background noise while preserving the volumetric, quantitative, and non-interferometric benefits of traditional Fourier ptychographic diffraction tomography (FPDT).
“DF-FPDT uniquely leverages non-matched illumination to selectively update only high-frequency components, enhancing fine structural details without the need for additional hardware or post-processing,” explains Professor Zuo. “This provides a powerful tool for live-cell imaging,maintaining the core advantages of FPDT while addressing the limitations posed by standard laboratory setups.”
The effectiveness of DF-FPDT was demonstrated through both computer simulations and experimental validation. The resulting 3D reconstructions exhibited significantly improved contrast and detail compared to images generated using traditional FPDT. Crucially, the technique proved capable of visualizing intricate subcellular structures and capturing dynamic events like mitochondrial fusion and fission in real-time, offering a clear and detailed view of cellular activity.
Professor Zuo envisions a broad range of applications for DF-FPDT. “DF-FPDT offers significant potential for widespread application in realistic laboratory settings,including drug screening,cellular analysis,and dynamic subcellular monitoring,opening new possibilities in biomedical research.”
The team is already looking towards future improvements, including integrating adaptive illumination and employing deep learning algorithms to further enhance image quality and speed. They also plan to develop a dual-mode imaging capability, allowing researchers to switch between contrast-optimized imaging and full refractive index reconstructions depending on their specific needs.
“These future enhancements will reveal even greater potential for DF-FPDT in a wide range of biomedical applications, accelerating discoveries in live-cell imaging and beyond,” concludes professor Zuo.
Source: Nanjing University of Science and Technology
Journal Reference: Ullah, H., et al. (2025). Intrinsic dark-field Fourier ptychographic diffraction tomography under non-matched illumination. iOptics. doi.org/10.1016/j.iopt.2025.100006