Vaccine Adjuvants: Unpacking the Science Behind Aluminum’s Role
Decades of Use in Enhancing Vaccine Efficacy and Safety Reviewed Amidst Public Scrutiny
Adjuvants, critical for boosting vaccine effectiveness, quality, safety, and longevity, have long relied on aluminum salts. These substances enable vaccines made from weakened or inactive components to stimulate a robust immune response, potentially reducing antigen amounts and required immunizations.
Understanding Aluminum Adjuvants
Often referred to as “alum,” the term historically denoted potassium aluminum sulfate. Modern vaccines, however, predominantly utilize aluminum hydroxide or aluminum phosphate, which possess distinct chemical properties and tissue persistence. These aluminum compounds have been integrated into vaccines for diphtheria, tetanus, acellular pertussis (DTaP), *Haemophilus influenzae* type b (Hib), hepatitis A and B, human papillomavirus (HPV), and pneumococcus since the 1930s.
Leading global health organizations, including the World Health Organization (WHO) and the U.S. Food and Drug Administration (FDA), endorse the safety and significance of aluminum adjuvants, establishing frameworks for their approval and quality assessment. While highly effective at promoting antibody production, aluminum adjuvants are less adept at stimulating strong T cell responses. Consequently, newer adjuvants like oil-in-water emulsions and Toll-like receptor agonists are employed in vaccines requiring more robust cellular immunity.
How Aluminum Adjuvants Work
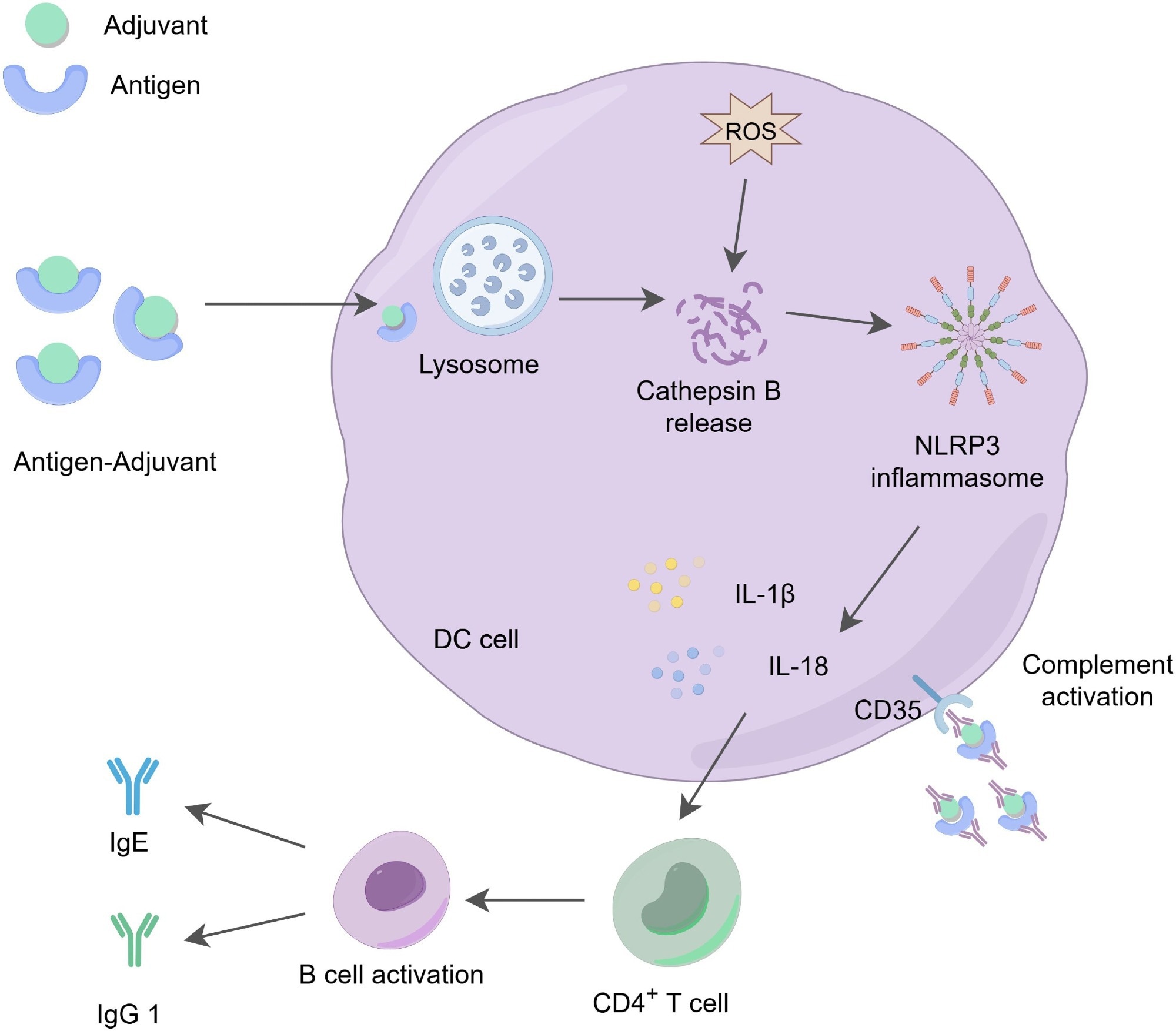
While the “depot effect”—the theory that aluminum salts create a reservoir at the injection site for slow antigen release—was once prominent, current research points to a more direct immune activation mechanism. Aluminum adjuvants actively recruit and trigger antigen-presenting cells (APCs), such as dendritic cells and macrophages, to the injection site. They induce lysosomal stress within APCs, activating the NLRP3 inflammasome. This activation prompts the release of pro-inflammatory cytokines like IL-1β and IL-18, crucial for regulating immune responses.
Aluminum adjuvants excel at driving T helper 2 (Th2) type responses, fostering B-cell activation and antibody generation, making them ideal for vaccines targeting extracellular bacteria and toxins. Their limited capacity for inducing T helper 1 (Th1) or cytotoxic T cell responses restricts their utility against certain intracellular pathogens.
Safety Profile and Body’s Handling of Aluminum
After intramuscular injection, aluminum salts gradually dissolve and enter the bloodstream, binding to transferrin before being cleared by the kidneys and excreted in urine. For individuals with healthy kidney function, the biological half-life of aluminum in the blood is less than 24 hours. The small amount of aluminum that remains is primarily deposited in bone, with minimal amounts potentially found in other organs like the brain. Aluminum phosphate adjuvants are processed more rapidly from tissues than aluminum hydroxide.
Concerns about aluminum levels in vaccines are often heightened, yet these doses are substantially lower than daily dietary intake. For example, an infant ingests approximately 117 mg of aluminum from baby formula by six months, significantly more than the cumulative 4.4 mg from the complete childhood vaccination schedule. Pharmacokinetic modeling by the FDA and other research bodies confirms that even this total exposure remains well below the minimal risk level set by the Agency for Toxic Substances and Disease Registry (ATSDR).
Local reactions, such as mild redness or swelling at the injection site, can occur but are generally minor and resolve on their own.1
Debunking Myths Surrounding Aluminum Adjuvants
Despite extensive scientific validation of aluminum adjuvant safety, public apprehension persists, often fueled by unsubstantiated claims linking them to chronic conditions like autism spectrum disorder (ASD), neurotoxicity, and autoimmune diseases. These misconceptions frequently stem from preliminary studies that correlate vaccine numbers with ASD prevalence or from animal research employing unnaturally high aluminum doses, designs that cannot establish causation.
Conversely, robust human cohort studies, including a 2025 Danish nationwide study of over 1.2 million children spanning 24 years, have found no evidence of increased risk for any assessed chronic disorders linked to early childhood exposure to aluminum-adjuvanted vaccines. Large-scale research consistently fails to support associations between recommended vaccinations and autism, developmental delays, or autoimmune conditions.4,10

Navigating Special Considerations and Future Directions
While the safety of aluminum adjuvants is well-documented, ongoing research explores potential sensitivities. A 2022 study indicated a potential association between cumulative vaccine-related aluminum exposure in infancy and the later development of persistent asthma.8 However, the authors stressed the need for further investigation due to the study’s observational nature and the possibility of confounding factors.
Infants with impaired kidney function and immunocompromised individuals are groups that receive particular attention regarding potential adverse effects.7,10
Aluminum adjuvants continue to be a cornerstone of modern vaccine development, demonstrating exceptional safety over nearly a century of use. Addressing public concerns and enhancing vaccine capabilities are driving the development of novel adjuvants. These next-generation options, including combination formulations and nanoformulations with Toll-like receptor agonists, represent the future of vaccinology, with licensed examples such as MF59, AS01, AS04, and CpG 1018 already in use for various vaccines.2,10
According to the Centers for Disease Control and Prevention (CDC), vaccine adjuvants are ingredients that help create a stronger immune response to a vaccine, making the vaccine more effective. They are rigorously tested for safety and are essential for protecting public health.

